Research
Our lab studies the role of mitochondrial dynamics and stress response pathways in cardiometabolic health. The prevalence of obesity and associated comorbidities, such as diabetes and cardiovascular disease, have increased dramatically over the last several decades. Activation of thermogenic adipocytes has emerged as a therapeutic strategy to increase energy expenditure and counteract weight gain. Recent evidence in mice and humans suggest a role for brown adipose tissue (BAT) in regulating the secretion of endocrine factors or batokines, such as fibroblast growth factor 21 (FGF21) and growth differentiation factor 15 (GDF15), that may promote cardiometabolic health by regulating energy homeostasis, glucose metabolism and cardiovascular function.
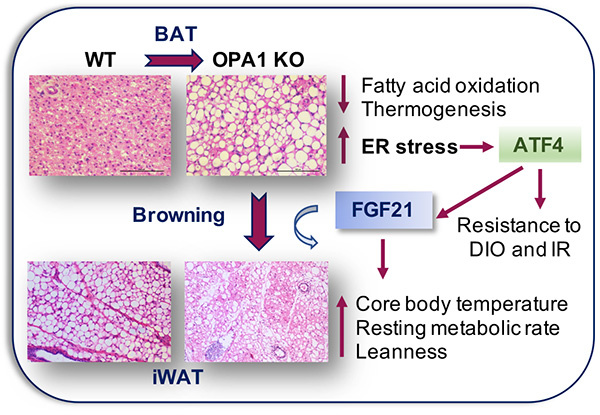
Our recent work in mice lacking the mitochondrial fusion protein optic atrophy 1 (OPA1) in BAT and in skeletal muscle demonstrated that mitochondrial stress induces the integrated stress response (ISR), which is required to improve metabolic fitness, partially via FGF21 secretion as a batokine. We also demonstrated that the ISR is induced in BAT in response to physiological stress, such as cold stress. Our research program aims to understand the role of mitochondrial dynamics and the ISR in the regulation of systemic metabolic adaptations in response to cold, diet-induced obesity, cardiac stress and mitochondrial dysfunction.
Our primary research projects focus on: 1. Investigating the role of PKR-like ER kinase (PERK) as an upstream regulator of ISR activation in BAT; 2. The role of the activating transcription factor 4 (ATF4), the main effector of the ISR, in BAT thermogenesis and metabolic homeostasis. 3. The role of GDF15 in the regulation of BAT-mediated metabolic protection. Additional projects in the lab are focused on investigating the role of mitochondrial dynamics and ATF4 in skeletal muscle in the pathophysiology of obesity and insulin resistance. We believe these studies have the potential of identifying new pathways that can be targeted to counteract obesity and its comorbidities.
Renata Pereira Alambert, PhD
Assistant Professor
Endocrinology and Metabolism
Dept. of Internal Medicine
FOE Diabetes Research Center
Molecular Medicine Program
Funding
Funding:
- University of Iowa Carver College of Medicine
- University of Iowa Fraternal Order of Eagles Diabetes Research Center
- National Institutes of Health (NIDDK)
Lab Members
Lab Members

Eric Weatherford, PhD
Research Assistant Professor, Endocrinology and Metabolism
Jayashree Jena, PhD
Postdoctoral Fellow
Endocrinology and Metabolism

Jennifer Streeter, MD, PhD
Associate
Cardiovascular Medicine

Luis Miguel García-Peña
Research Intern
Endocrinology and Metabolism

Marcelo Correia, MD MSc PhD
Clinical Assistant Professor, Endocrinology and Metabolism
Ayushi Sood
Rotation student
Jason Chen
Undergraduate Research Assistant
Cally Tucker
Undergraduate Research Assistant
Jason Gao
Undergraduate Research Assistant
Joshua Peterson
Undergraduate Research Assistant
Sanmati Thangavel
Undergraduate Research Assistant
Deeraj Manika
Undergraduate Research Assistant
Former Lab Members
Alex Marti, Research Intern
Kevin Kato, Research Intern
Sarah H. Bjorkman, MD, Fellow
Jivan Koneru, Undergraduate Research Assistant
Angela Nyunt, Undergraduate Research Assistant
lab photo 2022-1

2022
lab photo 2022-2

2022
lab photo 2021

2021
lab photo 2020

2020
Publications
Publications
Recent Publications
Pereira RO, Olvera AC, Marti A, Fang S, White JR, Westphal M, Hewezi R, AshShareef ST, García-Peña LM, Koneru J, Potthoff MJ, Abel ED. OPA1 Regulates Lipid Metabolism and Cold-Induced Browning of White Adipose Tissue in Mice. Diabetes. 2022 Dec 1;71(12):2572-2583. doi: 10.2337/db22-0450. PubMed PMID: 36170659.
Pereira RO, Marti A, Olvera AC, Tadinada SM, Bjorkman SH, Weatherford ET, Morgan DA, Westphal M, Patel PH, Kirby AK, Hewezi R, Bùi Trân W, García-Peña LM, Souvenir RA, Mittal M, Adams CM, Rahmouni K, Potthoff MJ, Abel ED. OPA1 deletion in brown adipose tissue improves thermoregulation and systemic metabolism via FGF21. Elife. 2021 May 4;10. doi: 10.7554/eLife.66519. PubMed PMID: 33944779; PubMed Central PMCID: PMC8128440.
Pereira RO, Tadinada SM, Zasadny FM, Oliveira KJ, Pires KMP, Olvera A, Jeffers J, Souvenir R, Mcglauflin R, Seei A, Funari T, Sesaki H, Potthoff MJ, Adams CM, Anderson EJ, Abel ED. OPA1 deficiency promotes secretion of FGF21 from muscle that prevents obesity and insulin resistance. EMBO J. 2017 Jul 14;36(14):2126-2145. doi: 10.15252/embj.201696179. Epub 2017 Jun 12. PubMed PMID: 28607005; PubMed Central PMCID: PMC5510002.
Pereira RO, Wende AR, Crum A, Hunter D, Olsen CD, Rawlings T, Riehle C, Ward WF, Abel ED. Maintaining PGC-1α expression following pressure overload-induced cardiac hypertrophy preserves angiogenesis but not contractile or mitochondrial function. FASEB J. 2014 Aug;28(8):3691-702. doi: 10.1096/fj.14-253823. Epub 2014 Apr 28. PubMed PMID: 24776744; PubMed Central PMCID: PMC4101649.
Pereira RO, Wende AR, Olsen C, Soto J, Rawlings T, Zhu Y, Riehle C, Abel ED. GLUT1 deficiency in cardiomyocytes does not accelerate the transition from compensated hypertrophy to heart failure. J Mol Cell Cardiol. 2014 Jul;72:95-103. doi: 10.1016/j.yjmcc.2014.02.011. Epub 2014 Feb 25. PubMed PMID: 24583251; PubMed Central PMCID: PMC4037364.
Pereira RO, Wende AR, Olsen C, Soto J, Rawlings T, Zhu Y, Anderson SM, Abel ED. Inducible overexpression of GLUT1 prevents mitochondrial dysfunction and attenuates structural remodeling in pressure overload but does not prevent left ventricular dysfunction. J Am Heart Assoc. 2013 Sep 19;2(5):e000301. doi: 10.1161/JAHA.113.000301. PubMed PMID: 24052497; PubMed Central PMCID: PMC3835233.
All Publications
See Dr. Pereira's complete list of publications.
Contact
Contact
Office Location:
4322 PBDB
169 Newton Rd
Iowa City, IA 52242
renata-pereira@uiowa.edu
Tel: 319-335-7962

